Get to know Sencilia – A Quick Sit-Down with Dr. Amar Kamat
Tiny, sensitive sensors replacing big, bulky and most of all costly equipment at a lower price? Impossible? Not according to Sencilia. Using an innovative fish-inspired technology, Sencilia harbors the vision of realizing ‘smart’ hospital wards by automating flow management in ICU’s with the aim of reducing intravenous dosage errors. This improves the accuracy of fluid balance in critical care patients, reduces the risk of adverse drug events that occur due to IV over/under dosage, and mitigates nurse workload by automating tedious measurements.
Almost exactly a year after the start of Sencilia, we had a quick sit-down with Dr. Amar Kamat, co-founder and managing director of the promising Groningen-based startup.
The Start
After finishing his master’s degree and his PhD in the United States, Dr. Kamat came to Groningen to do his postdoc where he mainly focused on 3d-printing and, you can guess it; bioinspired sensors. Together with his supervisor at the time, Asst. Prof. Dr. Ajay Kottapalli, he founded the company as we know now. The duo is complemented by their scientific advisors, Prof. Dr. Bayu Jayawardhana (University of Groningen) and Prof. Dr. Michael Triantafyllou (Massachusetts Institute of Technology), and a team of research and design engineers (Ir. Natanael Gomes and Ir. Felix Wood). The team’s technical expertise is complemented by colleagues at the UMCG who provide insights into clinical use cases and end-user needs, ensuring a clinical relevant end product.
During Prof. Dr. Kottapalli’s postdoc in Singapore and MIT he was already experimenting with using these sensors for intravenous infusion monitoring. After Dr. Kamat joined him in the Netherlands, the sensors were redefined to see where they could make the biggest difference. That’s where the first pieces were put together of what Sencilia is now. Successful feasibility studies followed after the team was awarded the NWO Takeoff Phase 1 grant in 2020, and after the green light of both Triade and RUG Ventures, Sencilia is now officially an early-stage startup.
The Product
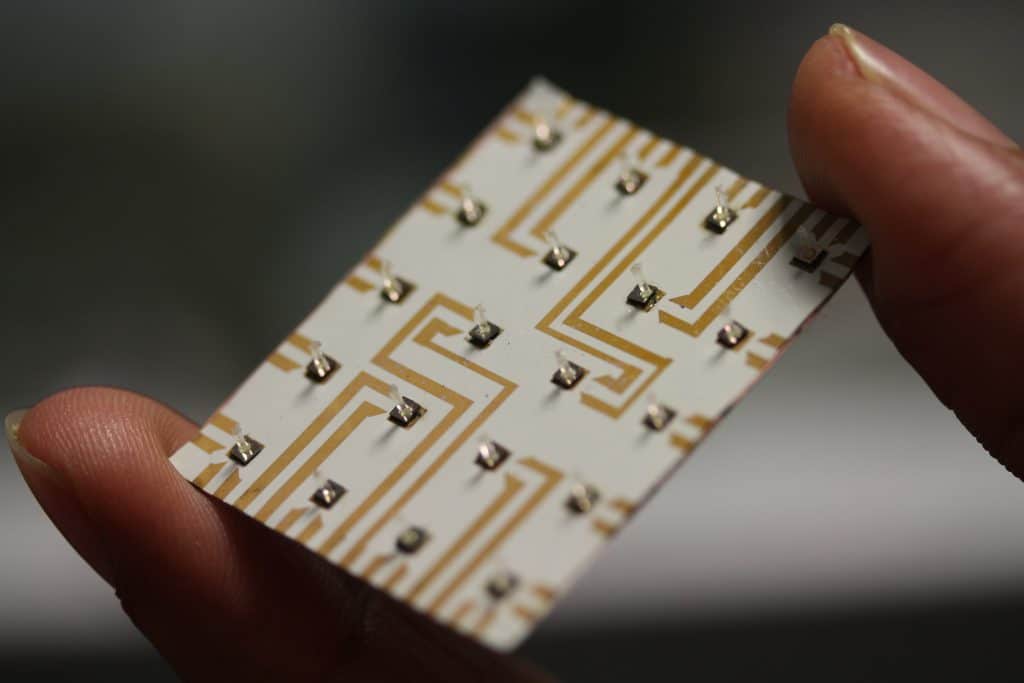
The name Sencilia comes from ‘Sense’ and the ‘cilia’ flow sensors fishes use for flow sensing. The sensors are inspired by fish’s sensing organs (known as the lateral line), responding to flow disturbances in their surroundings. These ‘cilia’ sensors form the basis for Sencilia’s sensors, making them capable of sensing very low flow rates while maintaining a small form factor.
Although the sensors can be used in a wide range of applications, Sencilia currently focuses on the biomedical field; mainly IV infusion monitoring. The IV flow measurements are currently done manually by nurses, making the process labor-intensive and prone to errors. As the sensors made by Sencilia are accurate, miniature, and low-cost, they seem to be the perfect solution for the automation of the process. This reduces the workload for nurses, resulting in more available time for patient care and ultimately better patient care via reduced adverse drug events. The technology is being developed in collaboration with the UMCG (Sencilia’s clinical partner), thus ensuring close involvement of the end-user (doctors and nurses) in the final product design and functionality.
But: IV flow management isn’t the only application. The sensors can be of value in countless settings, such as pipeline leakage monitoring, wind flow profiling, respiratory monitoring, airflow sensing and much more. The company already landed its first customer, CNR-INM (Institute of Marine Engineering, part of the Italian National Research Council), earlier this year, with whom they are collaborating to realize smart sensing solutions in offshore wind turbine monitoring and oil sloshing.
The Future
What’s next for Sencilia? For the company, the next step would be to reel in the next round of funding to help them further test and sound their sensors and grow. When looking at the product, Sencilia is currently very close to validating the concept via preclinical testing in the lab. The next step would be to test the sensors in something that resembles a living organism. Successfully completing such an experiment would take both the sensors and the company to new heights.
When asked where the company would be in 3 years, Dr. Kamat responded that he hopes to be fully ready to launch the product; ‘Three years, I think, is a legitimate amount of time for us to do all our clinical validation and get the regulatory certification’. In order to launch, the regulatory approval process would be the most important process. However, with Europe’s new Medical Device Regulations, it’s becoming harder and harder to reach the market. ‘But I think in three years, I hope that we will have already launched our first product, which is going to be flow sensors for infusion monitoring and critical care’. We can’t wait to see how the next few years will unfold for Sencilia!
Find out more about Sencilia here!